類天然環境中膜蛋白的熒光成像
Paper of the Month - #SMALPS
Your literary summer snack for September is here. Join us as we dive into the exciting realms of in vitro fluorescence studies of membrane proteins. Known to be a very challenging field, but new techniques are emerging.
The research was conducted in the Imperiali Lab, situated at the MIT, by Jean-Marie Swiecicki, Jordan Tyler Santana, and the namesake Barbara Imperiali, who are members of the departments of biology, physics and chemistry.
The challenging nature of membrane proteins
Membrane proteins are ubiquitous and play a crucial role in the cellular processes of all living systems (Fig. 1). They are the first point of interaction between cells and their surroundings since they are located at the cell's edge. As such, they mediate how cells sense and engage with their environment, converse with one another, and strictly regulate what enters and exits the cell. Not surprisingly, they are also the linchpin of the pharmaceutical industry. Although they represent about a third of the known human protein-coding genes, they are targeted by roughly 60% of the currently approved drugs. Unfortunately, at par with their biomedical value is the difficulty of characterizing them.
Their study proves to be challenging due to their hydrophobic nature and sensitivity to surrounding native environments. So far, traditional detergent-based methods often involved the detachment of these proteins from their native environment, which can alter their structure and function, and which is a time-consuming process. To address this issue, Swiecicki, Santana, and Imperiali introduce a strategic technique for fluorescence imaging that allows the visualization of membrane proteins while maintaining their native-like environment.
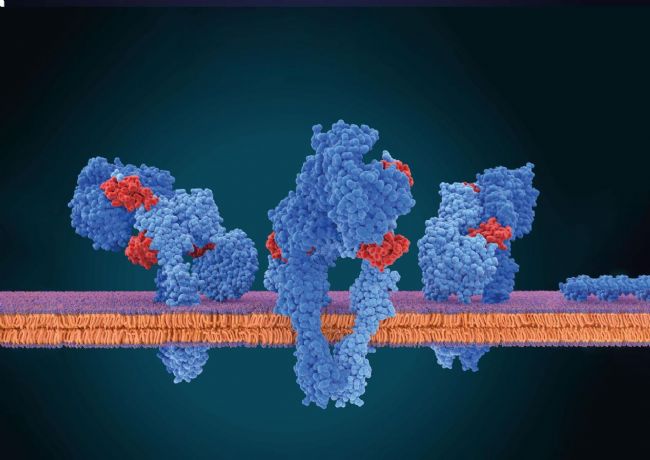
Fig. 1: Membrane proteins inside the cell membrane. Only this native environment ensures completely that the membrane proteins are folded correctly and thus are functional. Hydrophobic surface areas of the proteins are located inside the membrane while hydrophilic surfaces are located outside in either the ECM or cytosol.
Shine a light into native membrane environments
Their main strategy involved using a combination of genetically encoded non-canonical amino acids (ncAAs), bioorthogonal labeling, and detergent-free membrane protein solubilization based on an amphiphilic styrene-maleic acid polymer. Or in short: SMA. The researchers genetically incorporated ncAAs into the target membrane protein (Fig. 2).
Technical Dive
By repurposing a non-sense codon, most frequently the amber stop codon (TAG), ncAAs can be introduced. The inclusion of a generated tRNA synthetase/tRNATAG pair is necessary for this extension of the genetic code, known as amber suppression. As the fluorescent properties of ncAAs aren’t sufficient for single-molecule imaging applications, bioorthogonal labeling with a fluorescent dye compatible with single-molecule studies can help here.
These ncAAs contain specific chemical handles that can react selectively with fluorescent tags. By introducing these tags, which are compatible with living cells, they can achieve site-specific labeling of the protein within the cell membrane. All combined, this enables impressive single-molecule fluorescence studies.
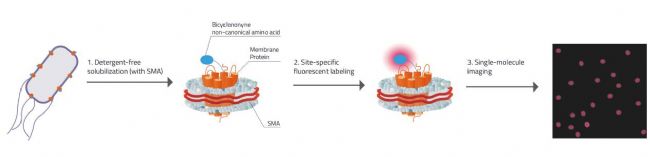
Fig. 2: Explanation on how Swiecicki's approach for Single-molecule imaging worked.
1. Detergent free solubilization & stabilization of the membrane protein inside the SMALP (SMA-Lipid-Particle / Nanodisc).
2. Membrane proteins have the unnatural amino acid Bicyclononyne incorporated that carries specific chemical handles that can react selectively with fluorescent tags.
3. Due to there only being one membrane protein molecule per nanodisc each signal is ensured to come only from one molecule.
In their paper Swiecicki, Santana, and Imperiali emphasize the importance of preserving native-like environments of membrane proteins to create profound research conditions. Hence, their approach aims to minimize the disruption to the protein's structure and function, enabling them to observe its behavior in a more realistic setting.
In summary, the paper introduces a strategic method for fluorescence imaging of membrane proteins within their native-like environment. This approach involves genetically incorporating unnatural amino acids with specific chemical handles, allowing for site-specific labeling of the protein using fluorescent probes.
This innovation has the potential to revolutionize the study of membrane proteins by enabling more accurate observations of their behavior and interactions within living cells. It might also result in a greater comprehension of their biological roles and the possible therapeutic applications that result from these discoveries.
Sources
· Swiecicki JM, Santana JT, Imperiali B. A Strategic Approach for Fluorescence Imaging of Membrane Proteins in a Native-like Environment. Cell Chem Biol. 2020;27(2):245-251.e3. doi:10.1016/j.chembiol.2019.11.008
Keywords: Unnatural amino acids, Bioorthogonal labeling, SMA copolymers, Membrane proteins, Fluorescence studies
Your literary summer snack for September is here. Join us as we dive into the exciting realms of in vitro fluorescence studies of membrane proteins. Known to be a very challenging field, but new techniques are emerging.
The research was conducted in the Imperiali Lab, situated at the MIT, by Jean-Marie Swiecicki, Jordan Tyler Santana, and the namesake Barbara Imperiali, who are members of the departments of biology, physics and chemistry.
The challenging nature of membrane proteins
Membrane proteins are ubiquitous and play a crucial role in the cellular processes of all living systems (Fig. 1). They are the first point of interaction between cells and their surroundings since they are located at the cell's edge. As such, they mediate how cells sense and engage with their environment, converse with one another, and strictly regulate what enters and exits the cell. Not surprisingly, they are also the linchpin of the pharmaceutical industry. Although they represent about a third of the known human protein-coding genes, they are targeted by roughly 60% of the currently approved drugs. Unfortunately, at par with their biomedical value is the difficulty of characterizing them.
Their study proves to be challenging due to their hydrophobic nature and sensitivity to surrounding native environments. So far, traditional detergent-based methods often involved the detachment of these proteins from their native environment, which can alter their structure and function, and which is a time-consuming process. To address this issue, Swiecicki, Santana, and Imperiali introduce a strategic technique for fluorescence imaging that allows the visualization of membrane proteins while maintaining their native-like environment.

Fig. 1: Membrane proteins inside the cell membrane. Only this native environment ensures completely that the membrane proteins are folded correctly and thus are functional. Hydrophobic surface areas of the proteins are located inside the membrane while hydrophilic surfaces are located outside in either the ECM or cytosol.
Shine a light into native membrane environments
Their main strategy involved using a combination of genetically encoded non-canonical amino acids (ncAAs), bioorthogonal labeling, and detergent-free membrane protein solubilization based on an amphiphilic styrene-maleic acid polymer. Or in short: SMA. The researchers genetically incorporated ncAAs into the target membrane protein (Fig. 2).
Technical Dive
By repurposing a non-sense codon, most frequently the amber stop codon (TAG), ncAAs can be introduced. The inclusion of a generated tRNA synthetase/tRNATAG pair is necessary for this extension of the genetic code, known as amber suppression. As the fluorescent properties of ncAAs aren’t sufficient for single-molecule imaging applications, bioorthogonal labeling with a fluorescent dye compatible with single-molecule studies can help here.
These ncAAs contain specific chemical handles that can react selectively with fluorescent tags. By introducing these tags, which are compatible with living cells, they can achieve site-specific labeling of the protein within the cell membrane. All combined, this enables impressive single-molecule fluorescence studies.

Fig. 2: Explanation on how Swiecicki's approach for Single-molecule imaging worked.
1. Detergent free solubilization & stabilization of the membrane protein inside the SMALP (SMA-Lipid-Particle / Nanodisc).
2. Membrane proteins have the unnatural amino acid Bicyclononyne incorporated that carries specific chemical handles that can react selectively with fluorescent tags.
3. Due to there only being one membrane protein molecule per nanodisc each signal is ensured to come only from one molecule.
In their paper Swiecicki, Santana, and Imperiali emphasize the importance of preserving native-like environments of membrane proteins to create profound research conditions. Hence, their approach aims to minimize the disruption to the protein's structure and function, enabling them to observe its behavior in a more realistic setting.
In summary, the paper introduces a strategic method for fluorescence imaging of membrane proteins within their native-like environment. This approach involves genetically incorporating unnatural amino acids with specific chemical handles, allowing for site-specific labeling of the protein using fluorescent probes.
This innovation has the potential to revolutionize the study of membrane proteins by enabling more accurate observations of their behavior and interactions within living cells. It might also result in a greater comprehension of their biological roles and the possible therapeutic applications that result from these discoveries.
Sources
· Swiecicki JM, Santana JT, Imperiali B. A Strategic Approach for Fluorescence Imaging of Membrane Proteins in a Native-like Environment. Cell Chem Biol. 2020;27(2):245-251.e3. doi:10.1016/j.chembiol.2019.11.008
Keywords: Unnatural amino acids, Bioorthogonal labeling, SMA copolymers, Membrane proteins, Fluorescence studies
標簽:
蛋白純化;膜蛋白
Copyright(C) 1998-2025 生物器材網 電話:021-64166852;13621656896 E-mail:info@bio-equip.com





